| Home | Research | People | Publications | Teaching | Opening | News | Contact |
|
Clean-Energy Engineering
|
|||
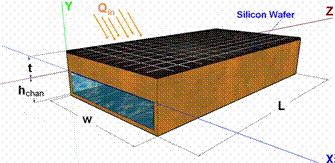
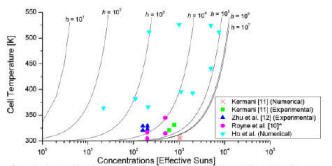
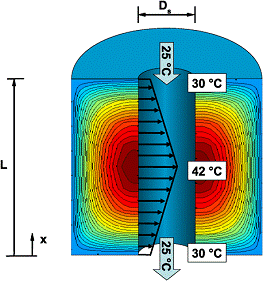
Concentrated solar photovoltaic systems are potentially cost-effective as they reduce the number of solar cells required to generate a given amount of electricity. This is done by focusing more solar radiation onto a given cell area, with concentration ranging from tens to thousands of effective suns. Solar radiation that is not directly converted to electricity remains as thermal energy, and excess heat increases cell temperature, which decreases solar-to-electricity conversion efficiency. Therefore, effective cooling is imperative for solar cells under high concentration. We developed a two-phase cooling strategy for concentrated solar photovoltaic systems [1,2], and our analysis indicated that the practical limits of solar concentration to be about 2000 suns for the six organic fluids examined, and 4000 and 6000 suns for water and ammonia, respectively.
[1] T. Ho, S. S. Mao, and R. Greif, “Improving efficiency of high-concentrator photovoltaics by cooling with two-phase forced convection.” International Journal of Energy Research, V.34, 1257 (2010).
[2] T. Ho, S. S. Mao, and R. Greif, “The impact of cooling on cell temperature and the practical solar concentration limits for photovoltaics.” International Journal of Energy Research, V.35, 1250 (2011).
|
|
||
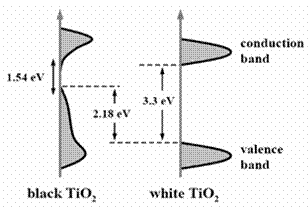
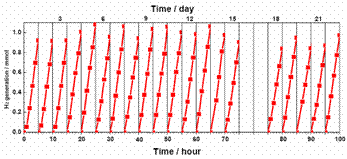
There is a considerable interest in the design of functional materials and devices for solar energy conversion, in anticipation of the renewable energy needs associated with limited resource of fossil fuels [1]. Of a range of materials that have been investigated in pursuit of greater utilization of solar energy, titanium dioxide is one of the most effective semiconductor photocatalysts, which has an electronic band gap in the ultraviolet wavelength regime. Narrowing the band gap is therefore vital to achieve efficient absorption of sunlight. While doping is a well-established method of tuning the band gap, its application to titanium dioxide has only limited success. We demonstrated [2] a conceptually different approach to enhancing solar absorption by tailoring disorders in nanophase titanium dioxide through hydrogenation. We showed that disorder-engineered titanium dioxide nanocrystals exhibited substantial solar-driven photocatalytic activity and are capable of producing hydrogen from water at room temperature. [1] X. Chen, S. Shen, L. Guo, and S. S. Mao, “Semiconductor-based photocatalytic hydrogen generation.” Chemical Reviews, V.110, 6503 (2010). [2] X. Chen, L. Liu, P. Y. Yu, and S. S. Mao, “Increasing Solar Absorption for Photocatalysis with Black, Hydrogenated Titanium Dioxide Nanocrystals.” Science, V.331, 746 (2011).
|
|
||
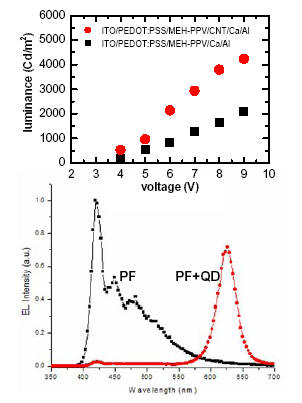
Organic light-emitting diodes (OLEDs) are attracting much attention due to their potential for solid-state lighting and flat-panel display applications. Although much effort has been devoted to the optimization of OLED performance, carrier injection barriers between the organic materials and the electrodes remain a limiting factor. For both polymer and small molecule based OLEDs, we demonstrated [1] that a carbon nanotube (CNT) layer can act as an electron injection enhancer at the cathode-organic interface. Along a different path, to achieve better spectral range and photo-stability, we developed [2] a proof-of-concept quantum dot-OLED hybrid device and demonstrated efficient energy transfer from the blue-emitting polymer to the red-emitting quantum dots.
[1] D. Liu, M. Fina, J. Guo, X. Chen, G. Liu, S. Johnson, and S. S. Mao, “Organic light-emitting diodes with carbon nanotube cathode-organic layer.” Applied Physics Letters, V.94, 013110 (2009).
[2] Z.-S. Guo, L. Zhao, J. Pei, Z.-L. Zhou, G. Gibson, J. Brug, S. Lam, and S. S. Mao, “CdSe/ZnS nanoparticle composites with amine-functionalized polyfluorene derivatives for polymeric light-emitting diodes.” Macromolecules, V.43, 1860 (2010).
|
|
||
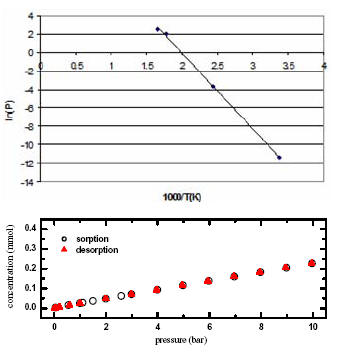
Hydrogen storage as a high pressure gas is energy and volume intensive, while storage in the liquid form requires extremely low temperatures. So solid-state hydrogen storage is attractive from the technological point of view, but has encountered tremendous challenges in terms of practical storage capacity and kinetics. MgH2 has been a model metal hydride for studying the fundamental processes related to solid-state hydrogen storage. Due to its high enthalpy of formation, bulk MgH2 generally does not release hydrogen below 300 oC, so there is a need to improve the thermodynamics of MgH2 such as reducing the enthalpy of formation. We developed [1] a free-standing nanoparticle film approach using Pd/Mg/Pd structure to improve hydrogen uptake and release kinetics of Mg nanoparticles as well as reduce enthalpy of formation. In addition, we are examining oxide porous materials with large surface area (over 1000 m2/g) and open porosity (more than 90% porous) for their potential as hydrogen storage media [2].
[1] S. Barceló, M. Rogers, C. P. Grigoropoulos, and S. S. Mao, “Hydrogen storage property of sandwiched magnesium hydride nanoparticle thin film.” International Journal of Hydrogen Energy, V.35, 7232 (2010).
[2] S. S. Mao and X. Chen, “Selected nanotechnologies for renewable energy applications.” International Journal of Energy Research, V.31, 619 (2007).
|
|
||
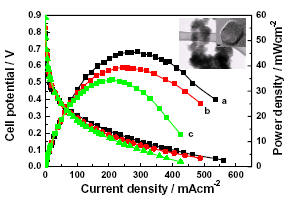
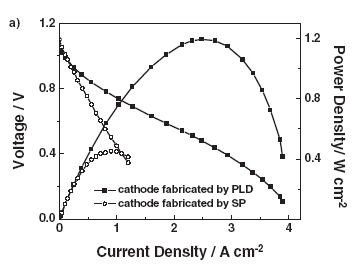
As clean energy conversion devices, polymer electrolyte membrane fuel cells (PEMFCs) have many advantages, including high energy conversion efficiency, which make them attractive for both mobile and stationary applications. While platinum (Pt) has been regarded as the most active catalyst for oxygen reduction reaction, the ultimate applications of PEMFCs are hindered, at least partially, due to the high cost of the noble material. We recently demonstrated [1] that highly crystalline CrN nanoparticles of fcc structure exhibit attractive catalytic activity and stability for the oxygen reduction reaction in PEMFCs. The result offers a possible solution to the need of cost-effective electrocatalyst alternatives for fuel cells. Additionally, we are also developing solid-oxide fuel cells (SOFCs) with perovskite-phase BaSrCoFeO (BSCF) thin film cathodes prepared on NiO-YSZ/YSZ composite substrates using pulsed laser deposition (PLD) and traditional screen-printing method [2]. The electrochemical performance of the SOFC cathode fabricated by PLD outperformed that based on the screen-printing method, which can be attributed to the smaller internal resistance within the cathode and the interfacial resistance between the cathode and the electrolyte. This work demonstrated the potential of PLD as a promising cathode deposition technique for achieving high performance SOFC. [1] H. Zhong, X. Chen, H. Zhang, M. Wang, and S. S. Mao, “Proton exchange membrane fuel cells with chromium nitride nanocrystal catalysts.” Applied Physics Letters, V.91, 163103 (2007). [2] B. Liu, X. Chen, Y. Dong, S. S. Mao, and M. Cheng, “A high performance nanostructured BaSrCoFeO cathode for solid-oxide fuel cells.” Advanced Energy Materials, V.1, 343 (2011).
|
|
||
There is a need of thermal management to control battery temperature as the trend to high power densities for vehicles would result in more heat generated within a smaller volume. A temperature increase of 15 oC could reduce the life of a Li-ion cell by 50%. Typically Li-ion cells have two electrodes separated by a thin membrane separator. When charging, electrons are conducted through the external electrical connection, and Li+ ions diffuse through the separator towards the negative electrode. When discharging, electrons and Li+ ions flow in the opposite direction. We studied through numerical modeling the effects of material and design modifications on the temperature distribution of Li-ion cells, such as the incorporation of an internal structure for better heat transfer, and the use of a carbon nanotube negative electrode and of separators made of Separion and different ceramics mixed with functioning particles. [1] M. Sievers, U. Sievers, and S. S. Mao, “Thermal modeling of new Li-ion cell design modifications.” Engineering Research / Forschung im Ingenieurwesen, V.74, 215 (2010).
|
|
||
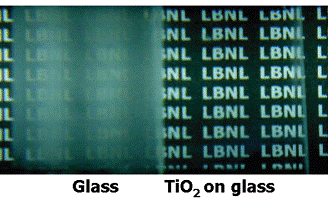
Since the discovery of extreme surface wetting phenomenon induced by ultraviolet (UV) photocatalysis, titanium dioxide has become the material of choice for environmentally friendly applications such as self-cleaning and anti-contamination coatings. Nevertheless, it remains a significant challenge to realize surfaces exhibiting persistent super-hydrophilicity but without the need of external stimuli, especially UV irradiation that also degrades the wetting surface over a period of time. We demonstrated [1] a porous titanium dioxide nanostructure that shows super-hydrophilicity without the need of light activation, and with stability against successive wetting-dewetting cycles. The wetting surface exhibits high optical transmittance over a wavelength range from near UV to the infrared, enabling practical anti-fogging technologies where transparency is critical. [1] V. Zorba, X. Chen, and S. S. Mao, “Superhydrophilic TiO2 surface without photocatalytic activation.” Applied Physics Letters, V.96, 093702 (2010).
|
|
||
| University of California at Berkeley |
this page is under construction |
||